Was Rude In A Way Crossword Clue, Exploding Basketballs In Public Prank!, 26.12 MB, 19:01, 1,822,864, D’Aydrian Harding, 2022-06-08T00:00:14.000000Z, 19, Mud City Crossword - WordMint, wordmint.com, 816 x 957, png, crossword wordmint, 20, was-rude-in-a-way-crossword-clue, KAMPION
In the covalent bonds featuring a large difference in the electronegativities of the bonded atoms, it is uncommon for the more electronegative atom to gain complete control over the bond pair of electrons, resulting in the formation of two ions. Here, the more electronegative atom forms an anion and the more electropositive atom becomes a cation. Electropositive radicals are atoms, ions, or molecules that can lose an electron and carry a positive electrical charge. An electropositive radical is formed due to the electropositive nature of a chemical species, which means a particular chemical species has the tendency to lose electrons in order to form positive radicals.
If the answer is d, then please explain what it would do. An atom becomes an ion (a) if it gains one or more electron (s) or (b) if it loses one or more electron (s). When it gains electrons it becomes negatively charged and is called an anion. When it loses electron (s) it becomes positively charged and is called a cation. The atom which loses electrons is called an electropositive element and losing electron it forms a positive ion called a cation. The atom which acquires electrons is called an electronegative element and accepting electron it forms a negative ion called an anion. Generally, metals are electropositive and nonmetals are electronegative. When a fluorine atom becomes an ion it has the same electron configuration as? The electron configuration of a f− ion is 1s22s22p6.
Electronegativity Bond Scale - Surfguppy - Chemistry made easy - visual
6.3.6: The Acidity of an Oxoacid is Determined by the Electronegativity
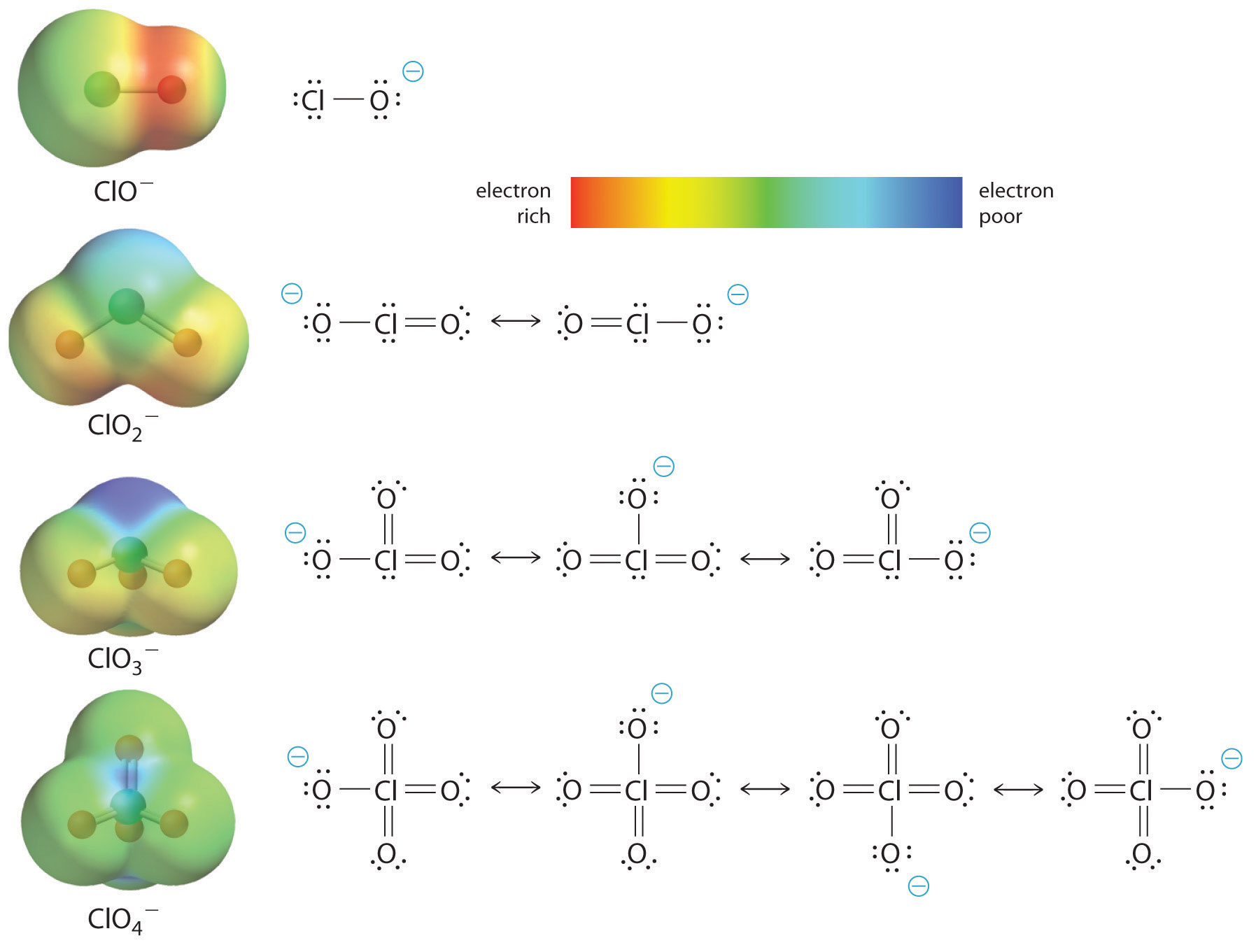
Octet Rule: Definition, Explanation, Exceptions and Examples
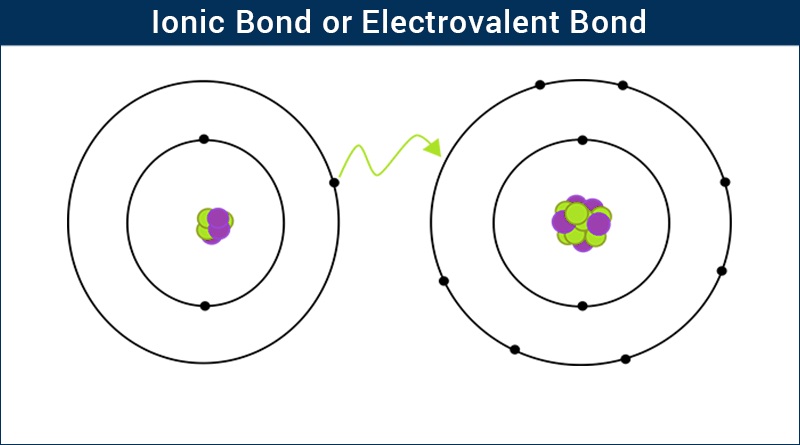
What is Ionic Bond - Surfguppy - Chemistry made easy - visual learning
Ionic vs Covalent Bonds

What is an ionic bond? | AmazingLife.Bio

Periodicity - Mr. Smith's Pre-AP Chemistry
An ionic bond is created between two unlike atoms with different

Real atomic radius and electronegativity.
Ionic Bond - Definition, Properties, Electronegativity & Examples with
Komentar
Posting Komentar